| | | Bioguard is Upgrading its GMP Facility to Human Level |
In the idea to bring better support to our customers in the international market, Bioguard is to upgrade its GMP (Good Manufacturing Practice) to the human level, since there is no regulation for veterinary diagnostic kits in Taiwan, thus, the Taiwanese government does not issue any official GMP for Veterinary diagnostic kits. In that regard, Bioguard is upgrading the facility in order to apply for GMP (Human standard), Bioguard signed a contract with Medical and Pharmaceutical Industry Technology and Development Center (PITDC), a government resolution for the application GMP Human-level...
|
| | | | |
| | | “We’re already operating as a GMP level for the production of our diagnostic kits, but since GMP certificate is unobtainable in Taiwan, with the urge of this approval in the market. we’ve found it very important to obtain the GMP certificate approval in order to bring better support to our customers and especially to overcome the strict regulation countries barrier that we’ve encountered in the market. with the focus on this project we expected to get the approved certificate at the beginnining of the
|
| | | | |
| 3rd quarter of this year.” Mentioned Mr. Edward Lai, President of Bioguard Corporation.
|
| | | | |
| Bioguard Invites You to Join the 3rd “Continuous Learning with Bioguard Class” |
Bioguard is glad to invite you to the third class of “Continuous Learning with Bioguard” on January 29, the class will be hosted by Dr. HUNG-SHI CHIOU, DVM, MVM, DCSVP, a specialist in Veterinary Pathology and Diagnostic Pathology. Dr. HUNG-SHI CHIOU will be discussing Diagnostic pathology in small animal practice…. Please register to reserve your place in the class at the following link https://forms.gle/7F1NJiAUeKJxNf1L6
“Continuous Learning with Bioguard” is a free online class brought to you by Bioguard Corporation |
| | AAFP Bans Elective Onychectomy at Cat-Friendly Practices |
The American Association of Feline Practitioners (AAFP) has announced an update for all designated Cat-Friendly Practices (CFPs): Elective feline onychectomy procedures are no longer allowed at these hospitals. The policy change aligns with the association’s2017 statement that strongly opposes onychectomy as an elective procedure. The ethically controversial procedure is rarely medically necessary and is already banned in many areas.The update went into effect on January 1, 2021, for all new CFP locations in North, Central, and South America. Going forward, it will also be standard in all practices seeking CFP designation. Current CFPs that continue to offer elective declawing procedures after July 1, 2021, will maintain AAFP membership but may not use the CFP designation.
The AAFP and International Society of Feline Medicine established the CFP program in 2012 as a global initiative to help make veterinary care less stressful for cats and their owners. Read more
|
| | | | |
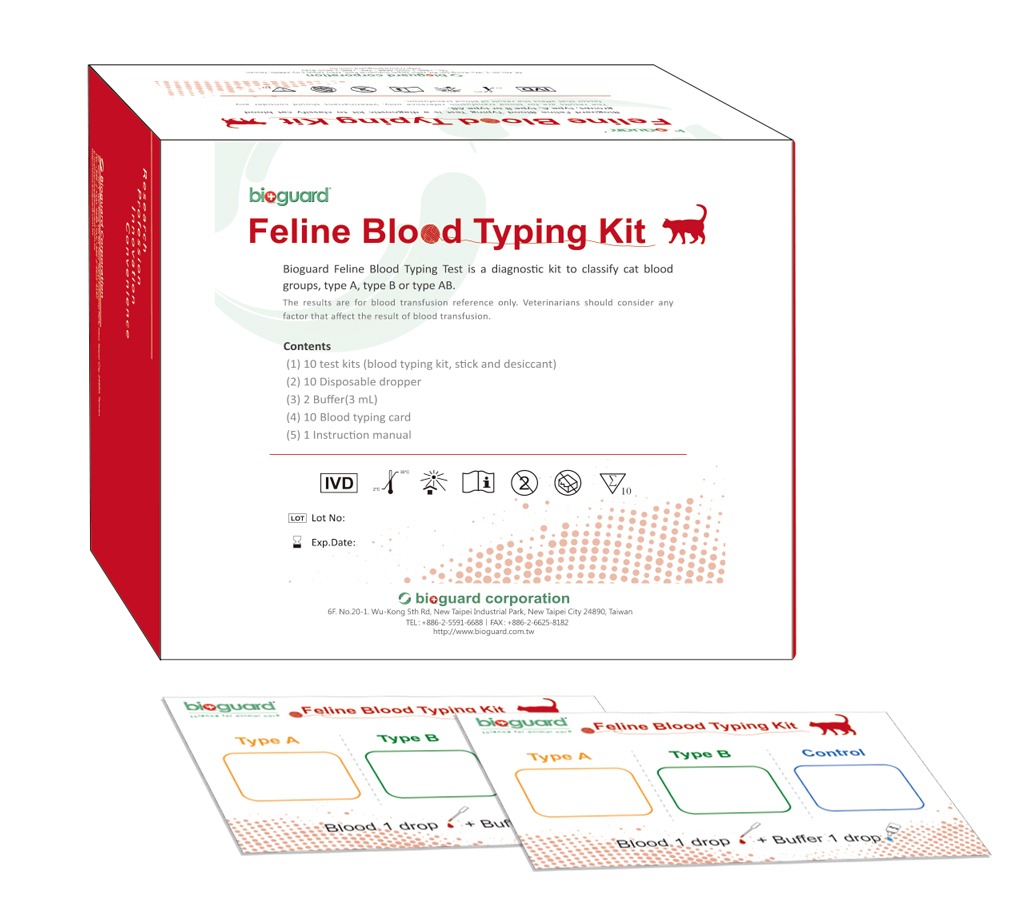
| Bioguard Feline Blood Typing Test is Based on Agglutination Reaction not in Antigen-Antibody Reaction |
| | | | |
| | | The blood types of cats are composed of mainly A, B, and AB. Type A is the most common, type B is rarer, and type AB is rarest. Type A erythrocytes express N-glycolylneuraminic acid (Neu5Gc), type B erythrocytes express N-acetylneuraminic acid (Neu5Ac) and type AB erythrocytes express both Neu5Gc and Neu5Ac. Instead of using antigen-antibody reaction for feline blood typing via monoclonal or polyclonal antibodies recognizing the antigens on erythrocytes, Bioguard’s “Feline |
| | | | |
| Blood Typing Kit” is based on the agglutination reaction that occurs when the blood sample interacts with the agglutinin/lectin in the test windows. Read more |
| | | | |
| About bioguard Corporation
The bioguard is a company focusing on animal disease diagnostic services and products.
Our animal health diagnostic center is the first and only ISO/ IEC 17025 accredited animal disease testing laboratory in Taiwan and China. Copyright © bioguard Corp., All rights reserved.
Our mailing address is: [email protected]
|
| | | | |
| | | | |